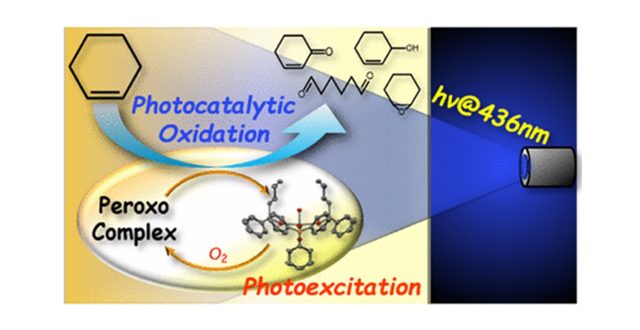
Oxygenation reaction of cyclohexene was studied under the presence of a fourfold UO22+ complex with cyclohexyldiphenylphosphine oxide, [UO2(OPCyPh2)4]2+, and blue light irradiation at 436 nm in acetonitrile. As a result, 1,6-hexanedial, cyclohexene oxide, 2-cyclohexen-1-one, and 2-cyclohexen-1-ol were photocatalytically generated as oxygenated products with turnover frequency = 6.7 h–1. In contrast, dimerization of cyclohexene was observed under Ar atmosphere. This implies that a hydrogen atom at the allyl position is abstracted by photoexcited *[UO2(OPCyPh2)4]2+ to give a cyclohexene radical and a U(V) intermediate [UVO2(OPCyPh2)4]+, being well supported by the density functional theory calculation. Under an O2 atmosphere, the former reacts with dissolved O2 to give 2-cyclohexen-1-one and 2-cyclohexen-1-ol. Dissolved O2 would be activated by the U(V) intermediate to afford O22– in the end, which drives oxygenation of the C=C bond of unreacted cyclohexene.
Visible-light Responsive Uranyl Complex Drives Photocatalytic Oxygenation
WRHI Newsおすすめ
Online published
(Laboratory for Advanced Nuclear Energy/Dr. Koichiro Takao and Dr. Satoru Tsushima)
“Photocatalytic Oxygenation of Cyclohexene Initiated by Excitation of [UO2(OPCyPh2)4]2+ under Visible Light”
ACS Omega, 2019, 4, 7194–7199.(DOI: 10.1021/acsomega.9b00635)
For details, click here
<Abstract>
