Although H+ has the largest hydration enthalpy among all the monovalent cations, we have demonstrated that anhydrous H+ can be crystallized together with selected diamidebuilding block (L) and NO3– even in acidic aqueous solutions, which were confirmed in three different structures. One of these anhydrous H+ adducts constitutes of H+-involved hydrogen-bond polymers [L···H+]n, which are coupled with another H+ adduct [O2NO–···H+···O–NO2]− as a counteranion unit. The anhydrous H+ can also be trapped between L and NO3– to form heteroleptic O···H+···O hydrogen bonds observed in two different crystal structures. DFT calculations revealed that there is no energetic barrier in these O···H+···O hydrogen bonds, having so-called a single-well hydrogen bond.
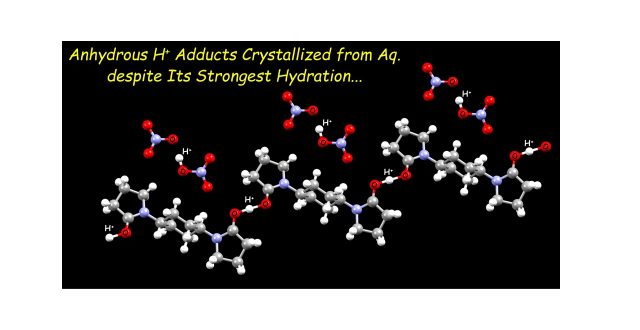
Unexpected Isolation of Anhydrous Proton Adducts from Aqueous System by Hydrogen-Bond Manipulation
WRHI Newsおすすめ
Online available
(Laboratory for Advanced Nuclear Energy/Dr. Koichiro Takao and Dr. Satoru Tsushima)
“Crystallization of Anhydrous Proton from Acidic Aqueous Solution with Diamide Building Block”
Cryst. Growth Des. 2019.(DOI: 10.1021/acs.cgd.9b01214)
For details, click here
<Abstract>
