M. Basile, et al., Chem. Commun., 2015, 51, 5306–5309, showed that a sodium ion is sandwiched by uranyl(VI) oxygen atoms of two 3 : 3 uranyl(VI)–citrate complex molecules in single-crystals. By means of NMR spectroscopy supported by DFT calculations we provide unambiguous evidence for this complex to persist in aqueous solution above a critical concentration of 3 mM uranyl citrate. Unprecedented Ca2+ and La3+ coordination by a bis-(η3-uranyl(VI)-oxo) motif advances the understanding of uranium’s aqueous chemistry. As determined from 17O NMR, Ca2+ and more distinctly La3+ cause strong O=U=O polarization, which opens up new ways for uranyl(VI)-oxygen activation and functionalization.
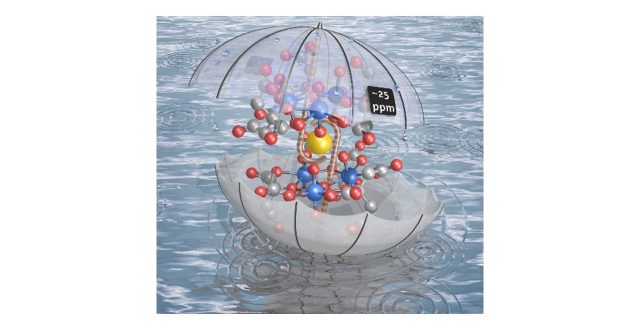
Uranyl citrate can selectively capture cations with specific ionic sizes
WRHI Newsおすすめ
Published
(Laboratory for Advanced Nuclear Energy / Dr. Satoru Tsushima)
“Trimeric uranyl(VI)–citrate forms Na+, Ca2+, and La3+ sandwich complexes in aqueous solution”
Chem. Commun.(DOI: 10.1039/d0cc05460g)
For details, click here.
<Abstract>
