Materials/Devices
Determination of the Distribution of Al on T-Sites in MFI-type Zeolite
Research Summary
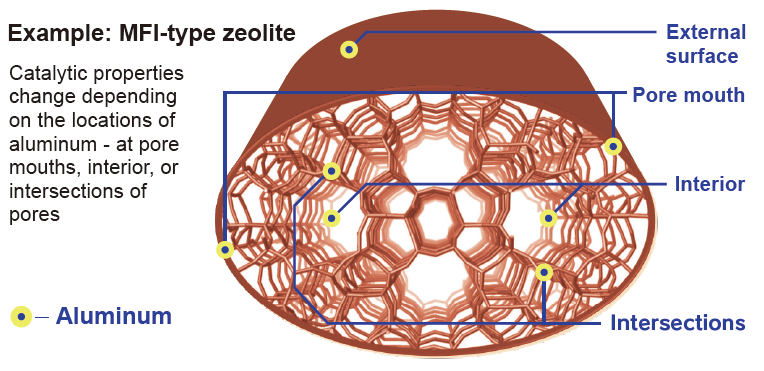
Zeolites are widely used as heterogeneous catalysts in industrial chemical processes because of their strong acidity and shape selectivities. The acidic properties of aluminosilicate-type zeolites originate from protons that balance the negative charge induced by framework Al atoms in tetrahedral sites (T sites). The catalytic properties of zeolites depend on various factors, including pore structure, acid strength and the amount of acid. Recently, the location and distribution of Al atoms in the zeolite framework have also been recognized as important factors for catalytic activity and selectivity due to their profound effect on the accessibility of molecules to acid sites and the spatial constraints of the reaction field in the pores. However, the relationship between the distribution of Al atoms in the framework and acid strength is not well characterized. While considerable effort has been devoted to estimate the distribution of framework Al atoms and their control within pores, this has proven to be a challenging problem limiting our understanding of zeolites.
Recently, we reported the controlled distribution of Al atoms during synthesis of the MFI-type zeolite. Charge imbalances within aluminosilicate zeolites result in the positioning of silica framework ((SiO4)4-)-localised Al3+ atoms near cations including inorganic cations, such as Na+ and K+, and/or organic cations, such as tetrapropylammonium (TPA+) as organic-structure-directing-agents (OSDAs). Because TPA+ species can be located at channel intersections, the Al atoms in ZSM-5 synthesized with only TPA+ in the absence of Na+ cations (ZSM-5 prepared in this way referred to as [TPA]) are preferentially located at intersections. Very recently we have found a new class of ZSM-5 zeolite characterized by the preferential localization of Al atoms to straight and sinusoidal channels using pentaerythritol (PET) in conjunction with Na+ cations ([PET+Na]). In this case, PET molecules occupy channel intersections, whereas Na+ species are found at straight and sinusoidal channels. Since PET molecules are different to TPA+ in that they have no charge, Al3+ species are instead located near Na+ species in this zeolite, resulting in a unique distribution of Al atoms throughout the MFI framework: Al atoms are observed at straight and sinusoidal channels, not at channel intersections.
We are now tackling the determination of the Al-Si distribution on T-sites in the MFI framework of Al-site controlled MFI zeolites based on a technique employing a Rietveld analysis of powder X-ray diffraction data.
![Fig. 1: Structure plots showing the position of the electron density maxima of NFS as refined. The green ball indicates the channel intersection as guide for the eye. (Left) the [TPA] sample is shown with a distribution of 10 sites over the pore system. (Right) the 5 sites in the [PET+Na] sample are shown avoiding the center of the IS, instead preferentially localizing to sites inside the connecting channels.](../../wp-content/uploads/2019/03/image2-1040x350.jpg) Fig. 1: Structure plots showing the position of the electron density maxima of NFS as refined. The green ball indicates the channel intersection as guide for the eye. (Left) the [TPA] sample is shown with a distribution of 10 sites over the pore system. (Right) the 5 sites in the [PET+Na] sample are shown avoiding the center of the IS, instead preferentially localizing to sites inside the connecting channels.
Fig. 1: Structure plots showing the position of the electron density maxima of NFS as refined. The green ball indicates the channel intersection as guide for the eye. (Left) the [TPA] sample is shown with a distribution of 10 sites over the pore system. (Right) the 5 sites in the [PET+Na] sample are shown avoiding the center of the IS, instead preferentially localizing to sites inside the connecting channels.


