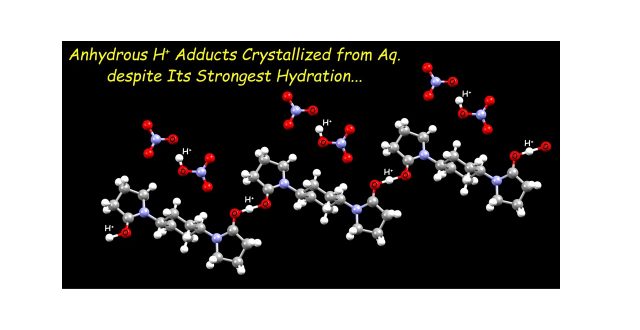
Although H+ has the largest hydration enthalpy among all the monovalent cations, we have demonstrated that anhydrous H+ can be crystallized together with selected diamidebuilding block (L) and NO3 – even in acidic aqueous solutions, which were confirmed in three different structures. One of these anhydrous H+ adducts constitutes of H+-involved hydrogen-bond polymers [L···H+]n, which are coupled with another H+ adduct [O2NO–···H+···O–NO2]− as a counteranion unit. The anhydrous H+ can also be trapped between L and NO3 – to form heteroleptic O···H+···O hydrogen bonds observed in two different crystal structures. DFT calculations revealed that there is no energetic barrier in these O···H+···O hydrogen bonds, having so-called a single-well hydrogen bond.
水素結合制御により水溶液系から無水プロトン付加物を単離
WRHIからのお知らせ おすすめ
先導原子力研究所 鷹尾康一朗准教授と津島悟特任准教授の共著論文
“Crystallization of Anhydrous Proton from Acidic Aqueous Solution with Diamide Building Block”
が、Cryst. Growth Des. 2019.にweb掲載されました。(DOI: 10.1021/acs.cgd.9b01214)
詳しくはこちら
<Abstract>
