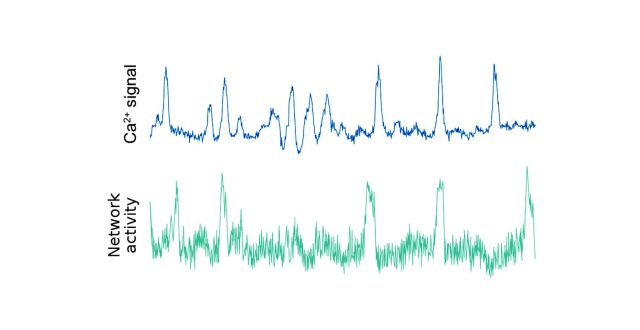
Residing in the islets of Langerhans in the pancreas, β cells contribute to glucose homeostasis by managing the body’s insulin supply. Although it has been acknowledged that healthy β cells engage in heavy cell-to-cell communication to perform their homeostatic function, the exact role and effects of such communication remain partly understood. We offer a novel, to our knowledge, perspective on the subject in the form of 1) a dynamical network model that faithfully mimics fast calcium oscillations in response to above-threshold glucose stimulation and 2) empirical data analysis that reveals a qualitative shift in the cross-correlation structure of measured signals below and above the threshold glucose concentration. Combined together, these results point to a glucose-induced transition in β-cell activity thanks to increasing coordination through gap-junctional signaling and paracrine interactions. Our data and the model further suggest how the conservation of entire cell-cell conductance, observed in coupled but not uncoupled β cells, emerges as a collective phenomenon. An overall implication is that improving the ability to monitor β-cell signaling should offer means to better understand the pathogenesis of diabetes mellitus.
【論文】β Cells Operate Collectively to Help Maintain Glucose Homeostasis
WRHIからのお知らせ おすすめ
バイオインタフェース研究ユニット Petter Holme特任教授とMarko Jusup特任助教の国際共著論文
“β Cells Operate Collectively to Help Maintain Glucose Homeostasis”
が、Biophysical Journalに掲載されました。
(DOI: https://doi.org/10.1016/j.bpj.2020.04.005 )
詳しくはこちら
<Abstract>
