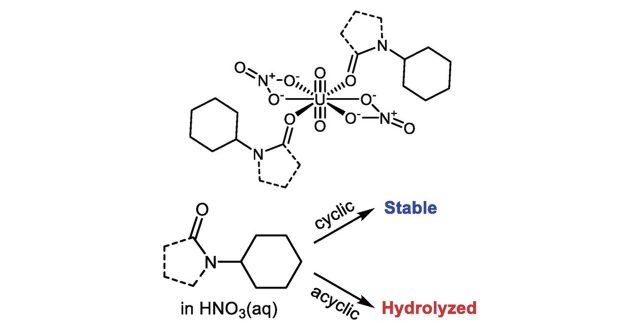
In a simple and versatile reprocessing method for recycling U and Pu from spent nuclear fuels, cyclic amides like N-alkylated 2-pyrrolidone derivatives (NRPs) are exclusively employed. However, there has been no convincing rational to explain why such a heterocyclic structure is required. To answer this question, we employed N-cyclohexyl-2-pyrrolidone (NCP) and N-cyclohexylformamide (NCF) as cyclic and acyclic monodentate amides, and focused on the following 3 topics in this study; (1) structural chemistry of their uranyl dinitrato complexes, (2) precipitation behavior of UO2 2+ from HNO3(aq) by using these amides, and (3) their chemical stability in HNO3(aq) simulating the reprocessing conditions for spent nuclear fuels. Fundamental coordination chemistry of UO2(NO3)2(L)2 (L = NCP, NCF) was found to be common to both L, regardless of the presence or absence of the pyrrolidone ring. Furthermore, both L exhibit comparable capability in precipitation of UO2 2+ from HNO3(aq). The most critical difference between NCP and NCF was found in their chemical stability in HNO3(aq), where NCF was gradually decomposed through acid-catalyzed hydrolysis, while NCP remained intact for at least 4 h. In conclusion, the pyrrolidone ring of NRPs plays an important role to sterically protect the carbonyl C from nucleophilic hydrolysis which initiates the amide C(=O)–N bond cleavage.
沈殿法に基づく使用済み核燃料再処理におけるピロリドン環の存在意義を解明
WRHIからのお知らせ おすすめ
先導原子力研究所 鷹尾康一朗准教授と津島悟特任准教授の共著論文
“Essential Role of Heterocyclic Structure of N-Alkylated 2-Pyrrolidone Derivatives for Recycling Uranium from Spent Nuclear Fuels”
が、 Bull. Chem. Soc. Jpn. に掲載されました。(DOI: 10.1246/bcsj.20200061 )
詳しくはこちら
<Abstract>
