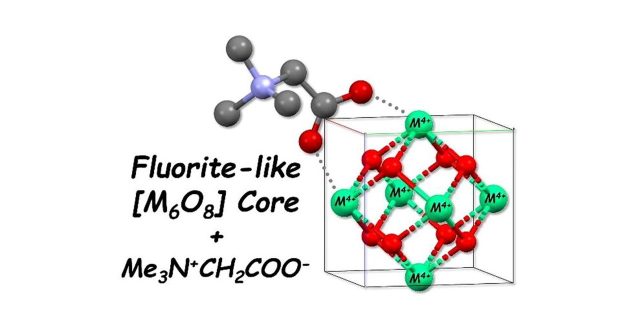
Tetravalent metal ions are hydrolyzed under presence of N,N,N-trimethylglycine hydrochloride (betaine hydrochloride, [Hbet]Cl) in aqueous solutions to afford [M6(μ3-O)4(μ3-OH)4(μ-bet)8(κ-bet)4(H2O)4]12+ (M4+ = Zr4+ (1), Hf4+ (2)) as hydrated perchlorate salts. These compounds were characterized by single crystal X-ray diffraction, elemental analysis and IR spectroscopy. As a result, we have found that fluorite-like [M6O8] coordination clusters are formed through octahedral arrangement of six M4+ linked by μ3-O atoms. Additionally, each pair of neighboring M4+ are connected by the μ-bet ligand through a syn-syn bridging coordination of its carboxylate moiety. This interaction seems to prevent further growth of the fluorite structure leading to formation of MO2. It was difficult to directly distinguish each μ3-O atom to be μ3-OH− or μ3-O2− due to its strongly anisotropic thermal displacement in the obtained structures. Bond valence sum analysis suggested that four μ3-OH− and four μ3-O2− are alternately arranged in the [M6O8] core motifs. Indeed, such a symmetric structure of [M6(μ3-O)4(μ3-OH)4] was confirmed in another phase of 1 at 296 K, where 1 transforms to a monoclinic structure (1′). The number of ClO4
− counteranions found in the structure determination is not enough to compensate + 12 charge of [M6(μ3-O)4(μ3-OH)4(μ-bet)8(κ-bet)4(H2O)4]12+ in any unit cells of 1, 1′ and 2. Instead, large solvent/ion accessible voids have been actually observed in their crystal structures, indicating that the missing ClO4
− are located therein and are significantly disordered to make them invisible in the crystallography. DSC analysis revealed that ClO4
− and H2O/H3O+ in the crystal lattice of 1 undergo unique structure relaxation and rearrangements pronounced by cold-crystallization to induce the phase transition from 1 to 1′ with elevating temperature.
アミノ酸誘導体を用いてZr(IV), Hf(IV)の加水分解に伴う蛍石型クラスター錯 体構造を制御
WRHIからのお知らせ おすすめ
ゼロカーボンエネルギー研究所 鷹尾康一朗准教授と津島悟特任准教授の共著論文
“”Fluorite-like hydrolyzed hexanuclear coordination clusters of Zr(IV) and Hf(IV) with syn-syn bridging N,N,N-trimethylglycine in soft crystal structures exhibiting cold-crystallization”
が、Inorg. Chim. Acta に掲載されました。(DOI: 10.1016/j.ica.2021.120622 )
詳しくはこちら
<Abstract>
